CALL 911 OR YOUR LOCAL HEALTH CARE PROVIDER IMMEDIATELY IF YOU SUSPECT SHELLFISH POISONING.
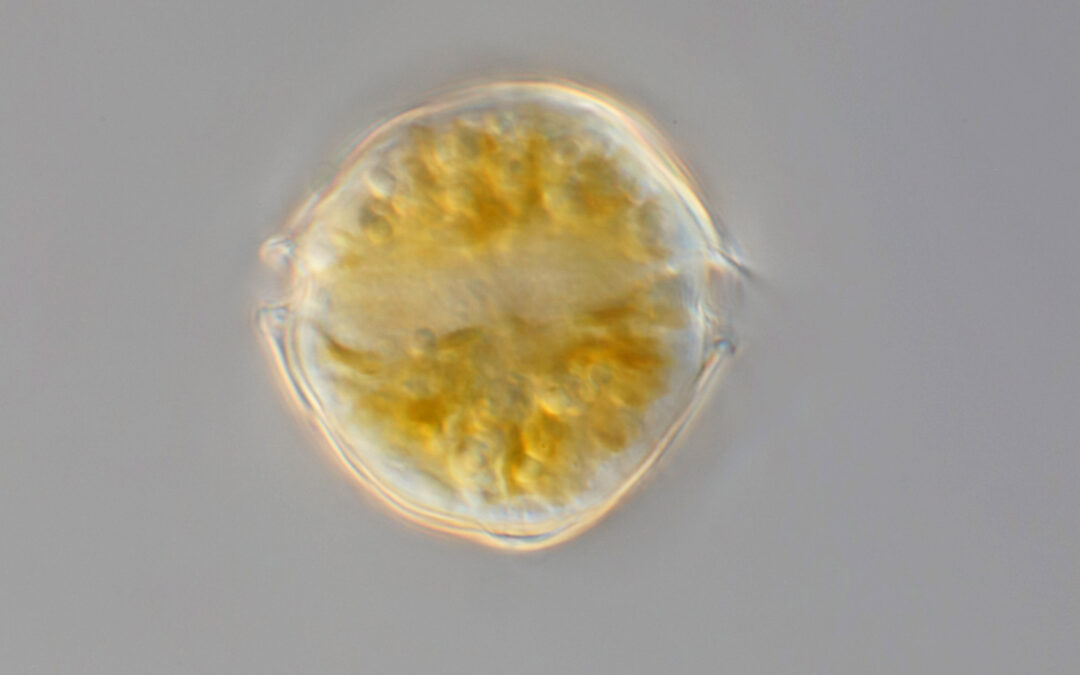
The Alaska Harmful Algal Bloom Network (AHAB) was formed in 2017 to provide a statewide approach to HAB awareness, research, monitoring, and response in Alaska.